Ep. 358 Lipid Masterclass: Impact of the Menopausal Transition with Dr. Thomas Dayspring
- May 4, 2024
- No Responses

I am excited to share our fourth class in our informative Lipid Masterclass series today, with the esteemed Dr. Thomas Dayspring. Dr. Dayspring is certified in internal medicine and clinical lipidology and is a distinguished fellow of the American College of Physicians and the National Lipid Association. He brings a wealth of expertise to our discussion today.
In this class, we dive into lipid and lipoprotein dynamics during the menopausal transition, exploring the impact of estrogen on gut health and its effects on laboratory findings. We look at the differences between hyper absorbers and hyper synthesizers, sharing clinical insights on routine lipid panels that can let you know if you are one of those individuals. We talk about Boston Heart Testing, highlighting the impact of specific biomarkers on brain health lipids and lipoproteins, and we get into the staggering differences between the half-lives of peripheral cholesterol and brain cholesterol. We discuss lipid permeability across the blood-brain barrier, highlighting those at risk for brain health concerns as they age, and we reveal strategies for managing lipid abnormalities. We also focus on LP(a), an ApoB lipoprotein with potent atherogenic and thrombotic properties, and its implications regarding calcific aortic stenosis.
I am sure you will find this enlightening conversation with Dr. Dayspring invaluable. There is one more class yet to come in this masterclass series. Be sure to listen in!
“If your brain suddenly started making too much cholesterol, and it got into neurons and started crystalizing, you would probably have some impairment of your neurons with many consequences, dementia being one of them.”
– Dr. Thomas Dayspring
IN THIS EPISODE YOU WILL LEARN
- How cholesterol absorption in the gut increases in perimenopausal women
- How hyper cholesterol absorbers tend to have elevated HDL cholesterol
- The impact of phytosterols on cholesterol absorption
- When should hyperabsorbers consider treatment?
- What research has shown regarding the differing effects of estrogen on brain health for older versus younger menopausal women
- What happens if a statin crosses the blood-brain barrier?
- How cholesterol synthesis relates to cognitive impairment
- The importance of understanding LP-PLA2 test results
- How Lp(a) levels in women tend to fluctuate, particularly during menopause
- Should women with heart disease consider hormone replacement therapy?
Bio:
Thomas Dayspring MD is a Fellow of the American College of Physicians and the National Lipid Association certified in internal medicine and clinical lipidology. After practicing in New Jersey for 37 years, in 2012, he moved to Virginia to serve as an educational director for a nonprofit cardiovascular foundation until mid-2019 as a Chief Academic Advisor for two major CV laboratories. Since then, he has served as a virtual cardiovascular / lipidology educator. Career-wise, he has given over 4000 domestic (in all 50 states) and several international lectures, including over 600 CME programs on atherothrombosis, lipids/lipoproteins (and their treatment), vascular biology, biomarker testing, and women’s cardiovascular issues. He has authored several manuscripts and lipid textbook chapters and performed several podcasts. For several years, he was an Associate Editor of the Journal of Clinical Lipidology. He received the 2011 National Lipid Association’s Presidents Award for services to clinical lipidology and the 2023 Foundation of NLA Clinician/Educator Award. He has over 34K followers on his educational Twitter (X) feed (@Drlipid). He has Gold Heart Member status as a professional member of the American Heart Association, and he serves as a Social Media Ambassador for the European Atherosclerosis Society and the National Lipid Association.
Connect with Cynthia Thurlow
- Follow on Twitter
- Check out Cynthia’s website
- Submit your questions to support@cynthiathurlow.com
Connect with Dr. Thomas Dayspring

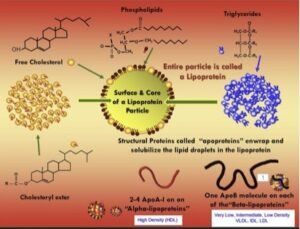
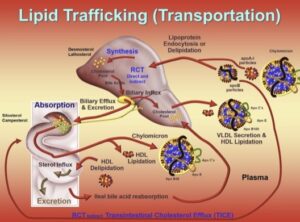
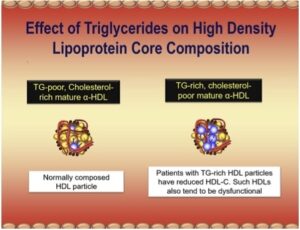
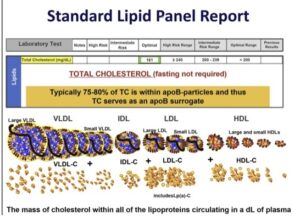
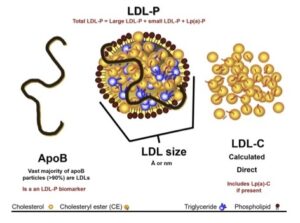
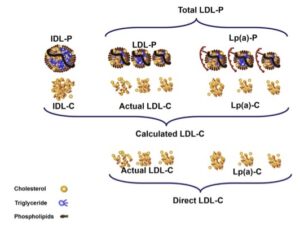
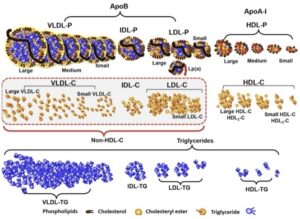

Transcript
Cynthia Thurlow: [00:00:02] Welcome to Everyday Wellness podcast. I’m your host, Nurse Practitioner, Cynthia Thurlow. This podcast is designed to educate, empower, and inspire you to achieve your health and wellness goals. My goal and intent is to provide you with the best content and conversations from leaders in the health and wellness industry each week and impact over a million lives.
[00:00:29] This is the fourth Lipid Masterclass Episode with Dr. Thomas Dayspring. And to recap, he is a fellow of both the American College of Physicians and the National Lipid Association and is a certified in internal medicine as well as clinical lipidology. He is a wealth of information and today, we focused in on changes in lipids and lipoproteins with the menopausal transition, the role of estrogens impact on the gut, and how this directly impacts our lab work. The differences between hyper-absorbers versus hyper-synthesizers, and particular clinical pearls on a regular lipid panel that can clue you in to whether or not you might be one of these individuals. We spoke about Boston Heart testing, looking at specific biomarkers, the impact of brain health, lipids and lipoproteins, the half-life of peripheral cholesterol versus brain cholesterol, and the differences are astounding, discussions centering around lipids and how they can cross the blood-brain barrier, those who are at greatest risk for concerns around brain health as they get older. We’ve reviewed options for addressing lipid abnormalities, and we spent a considerable amount of time talking about Lp(a), which is an ApoB lipoprotein, how it is incredibly atherogenic, has thrombotic properties as well as oxidized lipids and the changes that occur in our bodies as we’re navigating perimenopause into menopause. And lastly, we touch on how Lp(a) is implicated in calcific aortic stenosis, which is something I saw with great frequency in clinical cardiology. I know you will value this fourth conversation with Dr. Dayspring. We’ve got one more episode coming up.
[00:02:28] Well, Dr. Dayspring, pleasure to have you back on the podcast. I know that we had a very deep discussion into many topics specific to lipids on our last two episodes. But today, I would love to talk a little bit more about cholesterol absorption and some of the changes that you saw in your perimenopausal, menopausal female patients, that they were more prone to dealing with hyper-absorption of cholesterol at that stage of life. Could you walk us through some of the things that are happening physiologically in our bodies that are making this much more common and more predictable to see.
Dr. Thomas Dayspring: [00:03:06] Yeah. well, the absorption of cholesterol, and by absorption, we’re talking from the gut lumen into the intestinal cells, and then, of course, into the body is a super complex topic with many, many steps involved. And, of course, the transporters that internalize cholesterol are influenced by many things. When I worked in the labs down here, oh, geez, seven, eight years ago, [chuckles] maybe even a decade now, time flies, we had a lot of data at our fingertips. In fact, Health Diagnostics Lab had over 600,000 specimens where intestinal synthesis and absorption was determined. And, of course, we had genders on the patients and age. And we did see a trend when a woman entered menopause that there was a hyper-absorption of cholesterol from the gut.
[00:03:56] Now, that’s good. That’s shown to be pretty definitive. But if you want to know why? [laughs] I’m not sure I can give you an exact answer, why? Obviously, something that occurs during the physiological stage of menopause is influencing the transporters that regulate the absorption or actually expulsion of cholesterol from the gut back into the gut lumen. One would think that probably estrogen, to some degree, is involved with those receptors, but I don’t have a study that somebody’s actually looked at that and shown that or so, but hard to imagine what else it would be. And, you know, estrogen regulates tons of things throughout a woman’s body, but certainly is involved with lipid and lipoprotein homeostasis through all sorts of mechanisms on lipid synthesis, removal of lipoproteins from the plasma, etc. So, yes, the other group we found were hyper-absorbers of cholesterol also, well those who carry E4, ApoE4 allele.
[00:04:57] And again, I don’t have an exact reason as to why that should be so, but it seems to be. And we do know people with ApoE4 do tend to have higher LDL cholesterol levels, ApoB levels. So that’s probably part of the reason why they have increased lipid concentrations. But this is important because when women in the menopause transition are being evaluated cardiovascular wise, if you do happen to see elevated, especially LDL cholesterol, non-HDL cholesterol, or ApoB and it crosses a threshold where you think you have to intervene beyond lifestyle therapy, a very reasonable choice would be a drug that blocks cholesterol absorption, unless you are one of the practitioners, and that would be a minority who are actually measuring the sterile metrics that could help one confirm, am I dealing with a hyper-absorber or a hyper-synthesizer.
[00:05:49] Clearly, the latter would be if a drug is needed to be offered a stat or bempedoic acid. But a hyper-absorber, it makes great sense to try ezetimibe, of course, brand name Zetia in such a patient. So, it is very useful information. One little pearl and I don’t know how deep you want to get into the absorption of [laughs] cholesterol, but sometimes there’s something else in just the standard lipid panel that can tell you, you might be dealing with a hyper-absorber of cholesterol. They often have elevations of HDL cholesterol. And you would scratch your head and say, “Why would that be?” Not everybody has been taught that, when cholesterol leaves the gut and enterocyte, the enterocyte has to get rid of the cholesterol. And it does that by packaging it into an intestinal produced lipoprotein called chylomicron, which is sent into lymphatics and ultimately the systemic circulation. But the enterocyte also has the ability to efflux cholesterol directly into a very small HDL particle or ApoA1, which would help the maturation of an HDL particle. The particle would get bigger and carry more cholesterol. It’s no rule, don’t bet on it, there’s lots of people with increased HDL cholesterol who are not hyper-absorbers of cholesterol. But I always tell folks who aren’t doing the advanced testing that, look, if you see LDL cholesterol elevated and HDL cholesterol is also a little bit elevated, at least think of hyper-absorption of cholesterol, and that might influence you if you come to the decision, I need a drug to help improve those metrics.
Cynthia Thurlow: [00:07:24] Well, I think that’s a really valuable pearl, because there may be people listening who maybe their providers aren’t as familiarized with doing some of these advanced lipid tests, but they can look at an elevated LDL, elevated HDL and be thinking that they may be one of these individuals that’s a hyper-absorber. Now, you kind of alluded to the Boston Heart testing. So, if someone’s listening and they suspect that they themselves may be a hyper-absorber, they’re specialized testing through Boston Heart that has to be ordered through your provider and definitely through a provider who’s familiarized with these sterols. Help them understand, there are two sterols that would be elevated in people that are hyper-synthesizers and there were, I believe, a couple that were specific for the hyper-absorbers.
Dr. Thomas Dayspring: [00:08:78] Yes. Remember, cholesterol is a sterol. A sterol is a ring molecule that has some side groups sticking out, but there are numerous sterols. Cholesterol is the one we all know, it’s measured on everybody. Interesting, cholesterol is what we call a zoosterol. It’s the sterol that is produced in the animal kingdom. And I think last time we discussed, it makes up a lot of functions, but it’s in our cell membranes for the most part and it can be converted into certain hormones or vitamins or bile acids. Now, is there another living group of organisms that have cell membranes? Sure, plants. But plants don’t have cholesterol. There are a few that do, but the vast majority do not. But they have cell membranes just like everybody in the animal kingdom does too. So, the plant synthesizes things that look extremely similar to cholesterol.
[00:09:02] If you’re not a lipidologist and, you know, biochemistry would say, “Oh, that’s a cholesterol molecule.” No, but there are slight differences. So, because they are produced by plants, they’re called phytosterols and there’s 40 or 50 of them. It’s a gigantic group. Interestingly, animals make one sterol that’s going to serve all these functions and that’s cholesterol. But plants, and there’s a variety of plants, they make these phytosterols. So, most people eat plants. So if you swallow a plant, you’re not swallowing a lot of cholesterol. You might be if you’re eating other substances. But once it gets down into the small intestine, phytosterols are structurally very, very similar to cholesterol. So, there’s a receptor in the intestinal wall that has to bind to sterols, internalize them into the enterocyte just for completeness. That is called the Niemann-Pick C1-like 1 protein, NPC1L1.
[00:09:58] And it binds to sterols and it pulls them in. But these receptors, they’re unbelievably fine-tuned. So in humans, that receptor preferentially recognizes cholesterol and pulls it in. But since the phytosterols are kind of similar, it pulls some of them in too. Although the vast majority of the sterols it’s internalizing our cholesterol. So now we are humans. We don’t need phytosterols for our cell membranes. We don’t need phytosterols to make steroidogenic hormones. In fact, they could not be transformed into those they can’t make bile acids. And at very high doses, phytosterols, there are conditions where they go super high, they are actually toxic to humans and cause problems. So, of course, evolution made sure our intestinal cell really, by the end of the day, could say, “All right, your cholesterol molecule, you can come in. But hey, you phytosterols, sorry you got pulled in here, but we’re going to evict you right back to the gut lumen.”
[00:11:00] So there’s actually an efflux transporter in the intestinal wall. It’s called an ATP-binding cassette transporter G5 or G8, ABCG5 or G8, abbreviated. So, if cholesterol gets in, it’s going to be allowed to go into the enterocyte. It’s going to wind up in a chylomicron or an HDL particle as I alluded. But any phytosterol it gets in, if those ABCG5, G8 transporters are working, will be immediately evicted back to the gut lumen and be excreted fecally. Thus, if I measure phytosterols in the human blood, there should be, at best, infinitesimal trivial concentrations. But what if people have these transporters? They’re not exactly working perfectly, especially those ABCG5, G8. What if they fail to evict all of the phytosterols back to the gut lumen? Then those phytosterols are going to jump into the chylomicrons, be efflux to HDL particles, and all of a sudden be in our plasma.
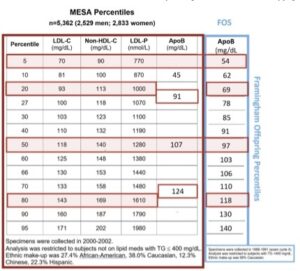
[00:12:03] So, if the lab is measuring phytosterols in the blood and they exceed a certain minimal level, we pretty much know, my God, if the intestine is over absorbing phytosterols, it’s super absorbing cholesterol. So, we actually use phytosterols as the biomarker that reflects intestinal synthesis of cholesterol. Now, I told you, there’s 40, 50 of these phytosterols. That would be some lab bill, if the labs were measuring all of them. They could, but they picked two of them and they picked the two most likely the ones that get in the easiest. And they are called sitosterol and campesterol. Some people whose sitosterol– no, cholesterol, sitosterol and the other is campesterol. C-A-M-P-E-S-T-E-R-O-L. Now, Boston lab reports the concentrations of those two phytosterols. They interestingly give you absolute concentrations, but they also use a ratio where they divide total cholesterol by those and they call that a normalized value.
[00:13:08] One should stay away from that. That’s an epidemiological tool that should not be used when trying to make a decision in an individual patient. You should look at the absolute concentration which is on their report. So, if I do see either sitosterol or campesterol are elevated, there’s no other causes other than hyper-absorption of cholesterol. Now, again, that’s cool to know, but if the patient doesn’t have a high LDL cholesterol, non-HDL cholesterol or ApoB, who cares? You’re not going to put people on drugs just because, hey, some phytosterols are getting in unless they’re at what’s called the 99.9 percentile. That’s the disease I sort of alluded to used to be called sitosterolemia, now it’s called phytosterolemia. That is a very dangerous disease. It’s only in one out of a million people. So, most physicians would never see a single case in their lifetime. Those running advanced lipid clinics get these type of rarities, referred to them, so they deal with them. And by the way, the current treatment is drown them with ezetimibe. Not the standard dose you or I might use in a run-of-the-mill patient, but really high doses of it. And before ezetimibe came around, these are not easy people to treat, so thank God that’s there.
[00:14:22] But so it’s measuring phytosterols in the blood are indications of hyper-absorption. In that study, I alluded to that where we looked at 600,000 people and menopausal women were hyper-absorbing. That’s what we looked at, campesterol and sitosterol, and we saw, boy, it’s a lot more elevated in women when they passed that age group and we used 51 as the most of those would be menopausal women. Probably not all, the vast majority, certainly so, because, as you know, on the lab form that a lab gets, it doesn’t tell the lab, is the woman menopausal or not? So, we use the age barrier to sort of assume we’re dealing with menopause. We had ApoE genotypes on everybody, so that was easy.
Cynthia Thurlow: [00:15:05] This is a really good point for people to understand and consider. You talked about the role of estrogen, and that is probably, perhaps contributing as women are transitioning into late perimenopause into menopause were making less endogenous estradiol, we become these hyper-absorbers in many instances of cholesterol. We can check it a couple of different ways. There’s the more advanced testing through Boston Heart, but you can also look at a traditional lipid panel looking at a high HDL. high LDL might be another indicator, another sense of something to kind of look more deeply at. And one big take home that I heard was, even if your markers on the Boston Heart, the campesterol and the beta-sitosterol are high, unless you also have correspondingly high ApoB and LDL, it’s not a clinical indication to treat that. I think that’s an important takeaway that I’m hearing from you.
Dr. Thomas Dayspring: [00:15:59] Absolutely. So, I see people tweet me or something, “Oh, my phytosterol level was a little high,” but they don’t tell me anything else if I refresh, geez, but did you get an ApoB or an LDL cholesterol? And if that comes back normal, who cares the type of concentrations we’re talking about that diagnose hyper-absorption are not going to hurt anybody. Like I said, “There is those in the 99.9 percentile, which are so rare they would hurt people.” But so, treatment of preventing atherosclerosis is get LDL cholesterol, non-HDL cholesterol, ApoB down. But if they’re not up, now, there are people who just do the lipid profile or ApoB first, and then if it’s out of whack, then do the sterol testing. We do them all at once. And this way you have that. It just otherwise you’re delaying.
[00:16:46] The other little pearl for the run-of-the-mill clinician too, is if you are dealing with a menopausal woman and she has a slight elevation of LDL cholesterol, non-HLD cholesterol, or ApoB, and you say, “Geez, I have to start lipid-modulating drugs because the lifestyle isn’t working.” Why not start Zetia or an ezetimibe instead of a statin? Repeat it in eight weeks the blood test. If it was a hyper-absorber, you’re going to see a nice improvement. If there was no response to Zetia, the odds are good you were dealing with a hyper-synthesizer. So, there’s many ways to skin a cat here. [chuckles]
Cynthia Thurlow: [00:17:20] No. And I love that because it’s something that we will talk about. I know that you have graciously offered to come back and we’ll do a kind of Q&A, I will then share with you my labs because I am a hyper-absorber and I actually take Zetia. And the differences in my lipids have been unbelievable. In fact, my functional medicine doc called me last week and said, “Holy cow, you are a massive hyper-absorber.” But he said, “Your numbers all look fantastic, but I will share all that with you so you can get a sense of what’s going on.” Let’s pivot a little bit and talk–
Dr. Thomas Dayspring: [00:17:49] Let me give you just one more little pearl on this-
Cynthia Thurlow: [00:17:51] Sure, of course.
Dr. Thomas Dayspring: [00:17:52] -for somebody like yourself. You know, one of the supplements that are often used to lower ApoB, LDL cholesterol are take phytosterol supplements, sitosterol, whatever they’re calling. And there are numerous products on the market. If you give phytosterols to somebody who is a hyper-absorber of cholesterol, they’re going to have serious hyper-absorption of phytosterols. And they do start to approach levels that you should be worried about. So one important, I think another reason to know is somebody hyper-absorber or not with the actual test is you would avoid that type of medicine and just get to the Zetia-
Cynthia Thurlow: [00:18:31] Yeah.
Dr. Thomas Dayspring: [00:18:32] -because the paradoxical thing is by getting phytosterols which compete with cholesterol for absorption, you actually lower the cholesterol getting in, but you increase the phytosterols getting in. So, the price you pay to use phytosterol supplements in a hyper-absorber of cholesterol is hyperphytosterolemia, which you’re not going to cause it to the 99.9 percentile. But I can, again, way more advanced lecture, there’s all sorts of data that at much lower thresholds, phytosterols are injurious to a variety of tissues.
Cynthia Thurlow: [00:19:06] Oh, that’s really interesting. And one thing about Zetia was years ago when it first came out, it was very expensive and so many of my cardiology patients needed to be on it. And I would just keep calling my drug reps to bring in samples so at least I could get them started. Now, it’s generic, so it’s very inexpensive, which for me worked out serendipitously. But for many people can be a big differentiator when we’re talking about trade versus generic drug therapy.
Dr. Thomas Dayspring: [00:19:30] Oh, yeah. And look like all branded drugs, when they come on, you’re going to pay for them until, and it takes many, many years before they transition into a generic drug. I think it’s 13,14, something like that. But interesting and of course, you would have never heard it from the manufacturer. But when they submitted Zetia to the FDA for approval, drug companies have to do all sorts of pharmacokinetic studies and dosing studies, and it turns out that actually even 2, 3 mg of Zetia a day significantly block absorption. Now, they settled on a 10-mg dose. They used that in most of their clinical trials, and the FDA said, “All right, you showed it safe, so we’ll go with the 10.” But I used to always tell people in the days you’re talking about when Zetia was still a branded drug, just take it every other day or every third day, and you could still get a pretty robust response if the patient was a hyper-absorber of cholesterol. The other thing you should assume, if you got no response to Zetia not dealing with a hyper-absorber, you’re looking at a hyper-synthesizer or other issue that’s causing the elevated ApoB.
Cynthia Thurlow: [00:20:37] Yeah, it’s also fascinating. Let’s pivot a little bit and talk about brain lipids and brain lipoproteins and how they can impact dementia risk, because anyone that’s a listener to this podcast, I think most if not all of us, are very concerned about cognitive health, especially as we’re navigating perimenopause into menopause and making sure that we’re doing as much as we can to help support our brain health.
Dr. Thomas Dayspring: [00:21:00] Well, brain health is, [laughs] you’re getting these super complex topics, [Cynthia laughs] and brain health involves a lot of things, of which lipids are one of things that affect the brain, but lots of other things going on upstairs. And of course, we talked about, “Geez, how might estrogen affect the absorption? It also affects the clearance of ApoB particles by influencing the LDL receptor.” But what does estrogen do anything to the brain? I think we all know it does. Almost come full circle for the longest while, it was always, “Oh, yeah, you take estrogen, your brain will stay healthy. Then, of course, some of those clinical trials started to come in, the Women’s Health Initiatives and there was, “Oh, boy, you may be aggravating dementia in some very complex study which looked at very old women, sort of in the middle women, and even a few perimenopausal women.” So it turns out, I think estrogen does different things to women who are 10, 15, 20 years into the menopause than it might do in a woman going through the transition or the early menopausal years.
[00:22:06] And I think we’ve come to the conclusion that’s when estrogen is super beneficial for the many attributes it might have in a woman or so, including cognition and mental functioning, depression whatever, at that stage of the game, whereas giving it to a more elderly woman, probably not going to get much bang for the buck and perhaps there’s some downsides to it also. So that’s come full circle. Now, how estrogen is given during the transition of early menopausal years is beneficially affecting the brain. You probably got to get a bunch of neurologists and everything here, but lipids are involved too. Even where the lipids are involved in the brain, I’m not exactly sure how they’re influenced by estrogen. So, I think it’s a given estrogen in the perimenopause, early menopausal years, unless there’s some contraindication to use it, it’s probably a wise thing for a woman to consider. You not only have to look at estrogen in the brain, but also the heart, the uterus, the breasts, every other part of her body. So choosing, “Hey, you’re an HRT candidate, requires a very serious evaluation of the total woman who’s confronting [unintelligible 00:23:14].”
[00:23:14] But lipids in the brain, what we don’t want to ever have is the development of a mild cognitive impairment or worsening impairment, full blown dementia as [unintelligible 00:23:24], even that, we have Alzheimer’s disease, which is the word everybody uses. But there are other causes of dementia that are not Alzheimer’s disease. And there is also atherosclerotic cerebrovascular disease that can contribute, if you’re getting little minor strokes here and there over time, sooner or later, you’re going to likely have some cognitive impairment or a frank dementia or so. So clearly that would be addressed, like you would be addressing coronary atherosclerosis. Get ApoB to control or whatever metric you’re using. If you’re not using ApoB, LDL cholesterol, non-HDL cholesterol, hopefully, and that would be a step in the direction for the ischemic brain issues doing it. But with Alzheimer’s disease, cholesterol is very much involved with that too.
[00:24:12] In fact, a long time, many years elevation of either LDL cholesterol or ApoB are associated with Alzheimer’s disease, also with the ischemic, the atherosclerotic disease. But it’s definitely a risk factor for Alzheimer’s disease. And we probably talked last time, cholesterol, every cell in the body, including the brain cells needs a little bit of cholesterol. So, what they make their cell membranes with. But cells don’t need a lot of cholesterol because cholesterol crystallizes and becomes toxic to a cell. So, the last place you want to have extra cholesterol would be a neuron up in your brain, because then the neurons are not going to start functioning as efficiently as they should. And one of the prices for that might be cognitive issues, or so. So the question would be, “If we lowered brain cholesterol, could we lessen the incidence of Alzheimer’s disease?”
[00:25:05] Now, look, step one is whatever cholesterol we’re measuring in the bloodstream. LDL cholesterol, HDL cholesterol has zero to do with the cholesterol that’s floating around your connective tissue in the brain. The brain totally synthesizes all of the cholesterol it needs. Your brain started doing that when you were inside your mother’s womb. That’s how quickly, you know, since cholesterol is so crucial for the brain, it starts making it in utero. Initially, some could come from mom’s lipoproteins too. But near the end of pregnancy, and certainly once delivery occurs, your brain is on its own and it synthesizes cholesterol seriously. It does not pull cholesterol from any of the lipoproteins floating in your bloodstream. And that’s why LDL cholesterol has zero to do with brain health or anything like that. And you see that all over the Internet, that type of nonsense.
[00:26:01] A little aside, and I’m giving you a lot of asides today, the half-life of a cholesterol molecule in the periphery. And when I say in the periphery, that’s any part in your body other than the brain, it’s on the other side of the blood-brain barrier. The half-life of cholesterol is a few days, and we make it, we excrete it, we absorb it. It doesn’t have to be preserved. The half-life of cholesterol in brain tissue is five years. That means when cholesterol is synthesized in the brain, it’s hanging on to it because it needs cholesterol and it has no other source of getting it. So not only does it make it, and it makes a ton of it in infancy, and by the way, in infancy your LDL cholesterol is 10, 15, 30 mg/dL. And those little brains are developing very quickly. Any mother or father knows how quickly the infants turn into functioning little adults there, because they have good brain function. So, your brain is just not looking, oh, boy, but if you have low LDL cholesterol, I won’t have a source of cholesterol. Nope. It makes it. So, we don’t ever have to worry about those little fallacies that you’re harming somebody or at least their brain by lowering LDL cholesterol too much. No relationship whatsoever.
[00:27:14] But anyway, you can imagine over time, if that brain suddenly started making too much cholesterol and that cholesterol got into neurons and started crystallizing, you’d probably have some impairment of your darn neurons. Be a lot of consequences, probably, but dementia being one of them. So, an interesting theory would come up with that is, “Hey, if there’s too much cholesterol in some brains predisposing brains to Alzheimer’s disease, maybe everybody should be on a statin to lower cholesterol in their brain.” Well, statins are the only lipid-modulating drug that we have right now that can cross the blood-brain barrier. So, when you do prescribe a statin, I mean, you wish it would just go to the liver and inhibit synthesis, the liver would grow LDL receptors, clear all your particles, and you’d be home free. But you don’t want statins getting into other cells per se inhibiting cholesterol synthesis.
[00:28:10] Luckily, with the doses of statins we prescribe, you’re not creating low cellular cholesterol in any cells in your body that’s hurting you. And if you really inhibit synthesis in the liver, you’re going to grow LDL receptors. The liver makes the most of them to clear your ApoB particle. But what if a statin that crosses the blood-brain barrier, by the way, in the old days, we used to think statins are in two categories, lipophilic, hydrophilic and hydrophilic and lipophilic was always thought to get through cell membranes a little differently with the lipophilic, because cell membranes are lipids can get into a cell easier than a hydrophilic statin. By the way, your only two hydrophilic statins are rosuvastatin and pravastatin, and all the rest are lipophilic. And it was thought that maybe those two statins would be a little safer not getting into the brain as much we now know, not true. All statins can get into the brain cells.
[00:29:03] So if extra cholesterol is part of the etiology of Alzheimer’s disease, it might seem that lowering a little bit of brain cholesterol synthesis would be good. But here’s a little something that popped up seven, eight years ago too. You and I can’t measure lipids in the brain. You’d have to do sort of stick a needle through your skull and whatever you suck out, measure lipids in it or so. But not that we would do it in the office, but researchers can take a spinal tap and get cerebral spinal fluid and measure lipids in there. And it tends to be a pretty good correlation with lipids in the cerebral spinal fluid and what’s going on in the brain. So, it turns out there are certain sterile markers that whatever they are in the cerebral spinal fluid, they correlate extremely well if you were measuring them in the plasma. So, we’re going to– there is a sterile marker that’s going to be of interest in this story I’m unfolding here. So, if I had an ApoE4 patient or I had a patient who maybe there was a terrible family history of cognitive disease and I was really worried, I maybe would want to suppress cholesterol synthesis in the brain a little bit, but I wouldn’t want to over suppress it. And here’s, why?
[00:30:13] There are two cholesterol synthesis pathways in cells. Evolution gave us redundant pathways. One goes through a bunch of sterol intermediates, the last one of which is called lathosterol and that is one of the Boston Heart lab markers. And the other is desmosterol, D-E-S-M-O-S-T-E-R-O-L. And that also is on the Boston Heart panel. Now, if you read the Boston Heart panel down the bottom, it tells you that the desmosterol pathway is a minor pathway. Lathosterol is the major pathway of cholesterol synthesis. Here’s what they don’t tell you. Yes, in the periphery, in the brain, the desmosterol pathway is the major pathway. So now these researchers came along and they measured desmosterol in the cerebral spinal fluid. And guess what, if it was low, it was a great predictor of mild cognitive impairment and Alzheimer’s disease. And that makes sense because if desmosterol is low, you’re not synthesizing enough cholesterol. And in the brain, you do need X amount of cholesterol or you’re going to have a deficiency of cholesterol. And according to this study, it would lead to mild cognitive impairment, dementia over time I mean, it doesn’t occur in two weeks.
[00:31:26] And the other beautiful thing that study showed is whatever the desmosterol concentration in the cerebral spinal fluid is, it’s the same in the plasma. So, you and I can look at desmosterol concentrations on the Boston Heart lab and have a good idea. Is it low? And if it’s low, I think you’d have to be a little bit worried that the brain is not synthesizing enough cholesterol. So look, if the person wasn’t on a statin, then that would be a marker this person is prone to cognitive impairment and Alzheimer’s disease. I don’t know what you could do about it, [chuckles] but you would get some neurological help. And there are therapies that preventive neurologists are using nowadays if they know, “Hey, you’re predisposed.” But I think if I had you on a statin, because the real reason I prescribed the statin is to get your ApoB down so you don’t get atherosclerotic disease in the periphery. I don’t want to pay the price of over suppressing brain cholesterol synthesis. So, if I have a person on a statin and I’m doing a follow-up ApoB, LDL cholesterol, I’m doing a follow-up desmosterol level too. I would have it at baseline and then I would follow up. And if it drops below what is called the 20th percentile, and there are well known reference ranges of these sterols, then I think, “You know what, that’s too much.” Maybe back off the statin a little bit.
[00:32:47] And the great news in the year 2024 is we have several other therapies that can get ApoB to goal without using statin. So, if you were an ApoE4 carrier, terrible family history of dementia, and “Hey, you’re also at risk for E4s or at risk for atherosclerotic disease big time and they do have high ApoB.” So, I would use a statin to a certain point. But if I saw I was suppressing desmosterol, then that shift from a statin. If I really got it below the 20th percentile, I’d back off on the dose a little bit. If you haven’t already added Zetia, I would. But we also have bempedoic acid, by the way, that only works in the liver. It doesn’t inhibit brain cholesterol synthesis. Or we have a PCSK9 inhibitor, monoclonal antibodies, which certainly do not cross the blood-brain barrier. So, in this select group of people prone to dementia, you can get ApoB to perfect levels without over suppressing cholesterol synthesis in the brain. So, it’s a fascinating topic.
[00:33:47] Now look, this is all conjecture. This is not backed up by giant randomized blinded trials, but it just makes a lot of sense. And if you start reading all the neurological articles on desmosterol deficiency in the brain, a lot of bad stuff that starts to happen. So, I think that’s a cool marker. What you’re really using, desmosterol, lathosterol for is in the periphery if you see somebody as a hyper-synthesizer and if you’re over synthesizing cholesterol, you’re going to be over synthesizing desmosterol and lathosterol. So, if those levels start to go up in the blood, you have a hyper-synthesizer. That’s an ideal patient for a statin. Normally we’d go to a statin first, bempedoic acid is a lot more expensive, tough to get approved by a third-party payer, but you would do them. But this is another reason, I believe, unlike guidelines, if you’re going to start a statin, unless it’s somebody who’s just had an acute coronary syndrome or something, don’t start the highest dose of a statin. Start the baby dose of a statin.
[00:34:49] Most of the LDL receptor upregulation with a statin comes with the smallest dose. Yes, if you double it, you get a few more LDL receptors, but the first dose gives you a 25%, 35% lowering, and subsequent titrations give you a 7%, 8% lowering. And of course, the higher the dose, the more likely other side effects of statins might appear. Not a lot of them, but they’re real, so you have to watch for them. So, I much prefer what I always call baby statins. That means the lowest dose of a statin. And if even that didn’t get you to go, look, I’m measuring the synthesis and absorption sterols, I would know, “Hey, this really needs Zetia,” or if it’s still whatever statin you prescribe, there’s still a lot of hypersynthesis, you can cautiously titrate it up, could add bempedoic acid if the patient’s rich and can afford it, but you could add a PCSK9 inhibitor too. Again, there are money issues there. Third party payers are not going to approve those drugs unless you have a lot of atherosclerosis or so. But at least textbook wise, there’s just a lot of tricks we can use. And measuring sterols helps you decide on this
Cynthia Thurlow: [00:35:56] I find it all really fascinating, and I do look forward to unpacking a bit more about the lipid-lowering drugs that are now available. As you appropriately identify, there are a lot of generic statins. Zetia is now generic and then we have these new kids on the block, these very potent drugs that not every insurance plan is covering unless you have failed other therapies or have extenuating circumstances. Now, one thing that we talked about in our last podcast together, but we didn’t spend a lot of time talking about, we talked about quite a bit about ApoB, and that is certainly a very important lipid marker. But I’d love to talk a little bit more about Lp(a).
Dr. Thomas Dayspring: [00:36:37] Sure.
Cynthia Thurlow: [00:36:37] This is something that I think is really misunderstood. What I love about it is that it can be a one and done test, meaning you don’t have to keep testing it. Certainly, I had my kids and my husband also tested because I think it can be very, very helpful for screening and helping people understand what is it testing for? What are we looking for, and what is the ideal range that you like to see as a clinician?
Dr. Thomas Dayspring: [00:37:00] Sure. Let me just correct you. An incy-wincy bit, ApoB is not a lipid test, it’s a lipoprotein test.
Cynthia Thurlow: [00:37:06] Sorry. Thank you.
Dr. Thomas Dayspring: [00:37:07] Lipid tests are cholesterol, triglycerides, blah, blah, blah. So, I’m a stickler on that so.
Cynthia Thurlow: [00:37:12] No, no. I appreciate that.
Dr. Thomas Dayspring: [00:37:13] The more we know, the correct terminology will likely stay on the tracks. So anyway, what is lipoproteine (a)? And the little a is just a small case a rather than a capital A. It’s not taught to most people, but lipoprotein capital A is an HDL particle, because HDLs use apoprotein capital A, so they wrap HDL particles. But the little a is something totally different. I always like to throw in a little history. It was actually discovered in the 1960s by a researcher called Kare Berg. And he was putzing around, he had some serum, and he was putting antibodies in there and see could I discover things that an antibody would bind to. And all of a sudden, in just a few people, an antibody was binding to something and he didn’t know what it was. So, what do antibodies bind to? Antigens. So, he called his discovery little a and the little a stood for antigen. So, of course, down the road a few years, they knew, this is an apoprotein. We’re going to keep calling it little a, but it comes from the word antigen.
[00:38:23] So what it is, it’s a member of the ApoB family of lipoproteins. So, it has nothing to do with HDLs, which are capital A particles. This is a little a particle, but this little a, which stands for apoprotein(a) or once it’s on the particle, it’s called apolipoprotein(a) actually binds to the ApoB that is already on the particle. But what is the ApoB family? If your listeners remember, it’s basically VLDLs, intermediate density IDL and LDLs. This apo(a) which is produced in only one spot your liver, once the liver makes it, it immediately jumps on what we call primordial beginning LDL particles in the liver cytoplasm, it does not bind to VLDL particles or IDL particles. So once apo(a) jumps on an LDL particle and it jumps on the ApoB moiety part of the LDL particle, it binds to it, and the initial binding is soft, but then there’s a more firm binding, so once it gets to that final binding, it’s stuck. So, the liver then secretes LDL particles that is manufacturing normally. But if your liver has made Lp(a) particles, it secretes these Lp(a) particles.
[00:39:45] So, you could have two types of LDLs coming out of your liver. If your liver is capable of making apoprotein(a). Unfortunately for most of our livers are not, but I don’t want to downplay it, because one out of five people have serious elevations of Lipoprotein(a). So, their liver sure makes it. In Blacks, it’s 1/3 and in Asians, it’s 1/3 especially Asian Indians. So, it’s a truly epidemic lipoprotein abnormality in those populations. But even look familial hypercholesterolemia, which most are taught about, that’s like one in 250 people. Lp(a) is one in five people. So if you go to a school, wherever you hang out with people, there are people walking around with Lp(a) and they have no clue, because study after study has shown us that most practicing physicians don’t know what Lp(a) is. Even a large amount of cardiologists don’t know what Lp(a) is. They never test for it. Even in people who have had heart attacks for goodness sakes that should be the first test to do when somebody’s had a heart attack, but it’s just not done.
[00:40:57] And the FH Foundation, now called the Family Heart Foundation is trying to do so much education on this. Look, there are drugs coming that’s going to abort this. And I think if and when they ever come, there’ll be a lot of education that pharma will be happy to pay for to get out there and teach doctors more about this. But in almost all of the guidelines that exist nowadays, they have transitioned into, say what you just said a little while ago, everybody needs an Lp(a) test once in their life. Now why only once? Because you either inherited the genes that makes your liver produce this peptide or you didn’t inherit those genes. If I didn’t inherit genes as a little baby, they’re not going to show up when I’m 40 years old [chuckles] those genes. So look, pediatric guidelines now suggest children get their first lipid panel by around the age seven or eight, sooner if there’s a family history of familial hypercholesterolemia. But that would also be the most opportune time to, “Alright, I’m going to do a lipid panel in this little boy or girl.” Let’s throw in an Lp(a) because it’s crucial to know that.
[00:42:05] The reason they want lipid testing early in life, because if you discover FH, you can start treatments when they’re kids. But look, if I get an eight-year-old who has high Lp(a), look, I’m not going to do anything to them at this time, but I would certainly caution their parents is this is some kid as you raise them, you want to stress cardiovascular health, exercise, proper nutrition, blood pressure, blah, blah, blah. Because the sooner you work on all of this, the sooner it can do. So, it turns out if apo(a) jumps on your ApoB and gets secreted as an Lp(a) particle, it’s way more atherogenic particle for particle than an LDL particle. LDLs are bad news, but you have to have a lot of LDLs before they start crashing your artery wall. The Lp(a) if it gets in that apo(a) has thrombotic properties, which is not good in your artery wall. But mostly the consensus now is, “Why did we even evolve apo(a) ? Did it ever have a person in our neanderthal man a long time ago or so, other than, hey, it’s a coagulant.” And in those days a lot of people bled to death. So, it was good if your blood was a little hypercoagulable, not so good nowadays.
[00:43:19] But oxidized lipid moieties now that’s a big word. Lipids can be what we call oxidized, especially those with double bonds are carrying oxygen molecules. If you’re a fireman, you know, oxidation means fire. So, if your lipoprotein particles are carrying oxidized lipids, they’re very injurious lipids. They create fires when they get into cells, they’re destructive in any tissue in your body. So, Lp(a) happens to be like a garbage truck for oxidized lipid moieties. So, one of theories is we evolved it, if there were oxidized particles floating around, they would go and bind to them and take them to a tissue, who knows where reticuloendothelial system, the liver where they could be disposed of and wouldn’t be so harmful. Who knows why? But oxidized lipids bind to a specific part of that apo(a) molecule. The ApoA molecule is a curlicue molecule. A lot of coagulation factors are. So, each of the little curlicues is called a cringle. Lp(a) has a variable number of these cringles, but there’s one specific cringle that the oxidized lipids attach to. And if they attach, they’re stuck there. So, unless that particle gets cleared, and right now, we really do not know how the body clears Lp(a) particles, but we do know if it winds up in your artery wall way worse than an LDL particle invading your artery wall.
[00:44:48] So we don’t like to see them because it’s a major contributor until maybe the near future it’s also one of these untreatable coronary risk factors, or it was always thought of in that way because we didn’t have specific therapies that lowered it, and we didn’t even have the proof that if you lowered Lp(a), you would have less adverse cardiovascular outcomes. Everybody assumes you would and there’s enough observational evidence and everything else to suggest that getting it low, if you have high is good, but it will take a definitive trial to prove that. And those trials are underway because X number of years ago, the researchers developed drugs that inhibit the hepatic synthesis of apoprotein(a) So if we can give those drugs and they’re safe, and you unfortunately inherited those bad genes, that tells your liver to make a lot of apo(a), you will stop making it. And if you stop making that, you can’t make Lp(a) particles.
[00:45:46] And the early data on these drugs is they reduce these things by 60%, 70%, 80%, even 90%, depending on what dose you use. So, there are actually clinical trials underway now, and there’s several in the pipeline. There’s one that got a head start called Pelacarsen that will stop your liver from making it. They’re checking different doses because one thing we have to decide is, “All right, if it’s good to lower Lp(a), do you have to blow it away or is just a little bit of a reduction good. And by the way, if you are blowing away apo(a), is there any harm to that?” None of that has shown up in the trials, the phase 1 and phase 2 trials so far. But until you test something thoroughly, you know, sometimes surprises occur. There have been any number of drugs that have made it through three, four years of clinical trials, and then all of a sudden adversity started showing up. So, our fingers are crossed the drugs that do inhibit apo(a) synthesis are these the genetic drugs, oligonucleotides antisense drugs that will, just by putzing with RNA and DNA, will prevent the synthesis of apo(a). And then you won’t make the Lp(a) particles, so they’re coming.
[00:46:57] So right now, if you showed up to me and you had a high Lp(a), I would certainly educate you that this is a bad news risk factor in most people. And right now, I’ve got nothing that at least is FDA approved to lower it. But I can advise you on every other aspect of good cardiovascular health. So again, nutrition, blood pressure control, please don’t smoke and ApoB lowering. Remember, if you have high Lp(a), your LDL family consists of two types of LDL particles, those carrying apo(a) and those not carrying apo(a). They would be your regular LDL particles. As bad as Lp(a) particles are, you have way, way, way, way more regular LDL particles, which is known to be causal for atherosclerotic heart disease. So, if I give you therapies that will get rid of your regular LDLs, lower ApoB, LDL cholesterol, non-HDL cholesterol, even though those drugs will be doing nothing to your Lp(a) concentration, you will get benefit. And that’s been shown in post hoc analysis of some of the statin and even PCSK9 trials.
[00:48:09] So right now, the guideline advice is if you think somebody is at risk for atherosclerosis because of high Lp(a), please lower ApoB as aggressively as you can. It’s an indication to start a statin in fact, in somebody who you think, “Oh, you’re low risk, but they have high Lp(a).” Statin therapy is suggested as a possible therapy right now. So lower ApoB as much as possible. Now, there will be a little noise for people who go [unintelligible 00:48:39] in it. Statins induce LDL receptors, which clear your regular LDL particles. LDL receptors do not bind to Lp(a) particles, so they can’t clear them. But since the overwhelming majority of your LDL particle family is the regular LDLs, statins can get rid of all of those. Even though you’re still left with Lp(a), you have mitigated atherosclerotic risk to a certain degree anyway. But there is some evidence and in some people it gets complicated. In some people, statins can increase the hepatic synthesis of apo(a).
[00:49:15] So some people, if they’re on Lp(a) and you say, “Hey, I’m starting you on a statin, because I want to get ApoB down.” ApoB goes down, but their Lp(a) level goes up a little bit, 10%, 20%, even 30%, it’s irrelevant. Because even in the studies, that is such a trivial increase in Lp(a) particles, you have so lowered the regular LDL particles that there’s very unlikely to be a downside. Just to give you numbers, a normal LDL particle count is 1200 nmol/L. A normal Lp(a) concentration is under 50 nmol/L. But if you have a nightmare Lp(a) concentration, it might be 150, 200 nmol/L. So, if you add up, hey, your total LDL particles would be 1200 LDLs and 200 Lp(a). The statins are going to get rid of all those regular LDL particles. So even that little bit of an increase, you’re still getting rid of so many adverse particles that there would benefit to a statin in that person. I’ll conclude therapy by your bank account is good, is there another lipid-modulating drug that has been shown to lower Lp(a)? Yes, by about 25% to 30%. And those are our PCSK9 inhibitors.
[00:50:36] Now, they have no FDA indication to treat high Lp(a) that would take a trial to show that. Yes, they do lower Lp(a), but you would have less heart attacks. And I don’t think the PCSK9 manufacturers are ever going to do that trial. Interestingly, when PCSK9 is involved with the once apo(a) is produced in the liver, PCSK9 traffics it over and attaches it to an LDL particle. So therefore, if you inhibit PCSK9, you will form make less Lp(a) particles. There’s some post hoc analysis in the PCSK9 trials that if you give PCSK9 people who have had an acute coronary syndrome, but they have high Lp(a), they have better outcomes than the people who didn’t have high Lp(a). But that’s all hypothesis generating. I think one of the reasons the PCSK9 inhibitor people aren’t going to do it is because we got so many more potent drugs coming that inhibit apo(a), so they’re not going to waste their money doing a billion-dollar trial to show you PCSK9 and once these other drugs come out, if they work and they’re safe, nobody’s going to be using PCSK9 inhibitors.
[00:51:42] But right now, you could. So, if you had a person who was worried about high Lp(a), maybe you’ve done a coronary calcium, a CCTA, and wow, they have atherosclerosis, and you can’t get that ApoB down to lower or even if you do get it as low as you want and you say, “I’m going to take a gamble at lowering Lp(a) works, you could, if they can afford it, give them a PCSK9 inhibitor.” So, very interesting there, and time will tell. So for now, some people, mostly the wealthy, are using PCSK9 inhibitors because your third party payer will not approve it, because it doesn’t have an FDA indication to do that. So we did mention, “Hey, it’s one time in your life.” Over the last year, there’s been some data and the reason why they said that is because you’re either making it or you’re not. Whatever concentration you have, it’s pretty much going to be that it’s at a mature population by the age of five. So technically, your age seven Lp(a) will be your age 57 Lp(a).
[00:52:45] Now, you never measure Lp(a) during an acute situation, an infection, some other crisis, because it’s what we call an acute phase reactant. Your liver makes a lot of proteins when the house is on fire, so to speak, and it will produce extra apo(a) because it’s churning out all sorts of proteins. So, you never measure Lp(a) in a sick person. You shouldn’t be doing lipid profiles in people who are otherwise ill for goodness sakes. So, you don’t do it there. But there’s been, part of it is every damn lab does their own assay for it. So, yeah, you should stick with the same lab. So, there have been instances where a person’s Lp(a), they checked it 10 years later and it was a little higher than it was. Now, is that a different assay that happened or can Lp(a) fluctuate a little bit? And the one area that I know you’re very interested in is at menopause Lp(a) tends to go up. So, whatever your Lp(a) is prior to menopause, it might be a little higher afterwards, which certainly tells you estrogen involved, but we don’t know how.
[00:53:53] But the other time, Lp(a) goes through the roof. Most of women are not checking it during pregnancy, but very high, and they have very, very high estrogen levels during pregnancy too, whereas in menopause, they have low estrogen levels, but it still goes up. So don’t ask me to explain that to you. It’s so, so high in pregnancy, but when you lose estrogen in menopause, it likewise goes up. Here’s my belief. Somebody’s going to have to do a big trial to adjudicate that. But these studies that are showing, “All right, premenopausal, her Lp(a) was this, and postmenopausal, it’s this.” I believe that if that woman had a normal Lp(a) level prior to menopause, let’s say under 50 nmol under 30 mg/dL and it goes up after menopause, it goes up a trivial amount. So, if it was normal to begin with, it might be a little higher after menopause, but it’s still normal. Likewise, say you had a high Lp(a) before menopause, so it’s going to go up a little higher, but does it matter if your Lp(a) was 150 and it goes to 170? They’re both high risk Lp(a)s so I don’t think most people will go from a normal Lp(a) prior to menopause to a disaster Lp(a) after menopause. But especially since drugs are coming along, if any woman wants to repeat it after menopause, be my guess, it tends to get repeated a lot too, because sometimes the patients don’t even know they had it checked earlier in life, or they’re with new doctors who don’t know them and they don’t have their old records with them. So, this is like your blood type. You want to write down your Lp(a).
[laughter]
Cynthia Thurlow: [00:55:28] That’s a good comparison.
Dr. Thomas Dayspring: [00:55:30] The last thing I’ll say is, you already did it. But when you check your Lp(a), you have other loved ones in your life, so they also deserve a one-time Lp(a) test. God forbid, you had high Lp(a). I would call it child abuse if you didn’t get your kid checked for Lp(a). But everybody gets it once, then you can either deal with it and if they’re kids now, by the time they’re grown-ups, they’ll have all likely these other wonderful therapies that will be available to it. So, get it tested. And if you get it tested or if you go to your doctor and ask, “Can I have an Lp(a) test?” And your physician or clinician says, “Nah, you don’t need that. Nobody believes in it.” I always joke, run out of that office as fast as you can and find a knowing provider. My goodness, it’s in all the guidelines now. How can a practicing clinician not understand this for goodness’ sake? So, you got to be your own advocate with some we talked about that for so many medical conditions and that’s another one to do.
[00:56:31] Last thing, it’s not only this nightmare risk factor for atherosclerotic heart disease. It’s probably the second most common cause of calcific aortic stenosis right now. So, these Lp(a)s can invade your aortic valve leaflets. One of the attributes of the apoprotein(a) is it’s a bone forming molecule. [chuckles] It turns on bone forming molecules and stuff. So you, as a cardiovascular nurse, know you don’t want calcium in your aortic valve, because one day somebody’s going to have to fix that valve. So if you know, “Oh, goodness, this person has high Lp(a), you want to keep a close eye on any aortic valve as they age.” You don’t wait for the systolic murmur to appear when you got more severe echoes periodically. Just do it.
Cynthia Thurlow: [00:57:18] Yeah. And an ultrasound of the heart is noninvasive. It’s fairly inexpensive, it’s an easy screen. The one thing that I would ask, because of course, it’s in the back of my head, the women that are taking hormone replacement therapy within that ideal five-year window of making the transition into menopause, is that providing protection for some of these markers in your clinical experience? I’ll just kind of put it that way.
Dr. Thomas Dayspring: [00:57:44] Yeah. Look, my clinical experience, I wasn’t doing a long term follow ups on patients who’s getting a heart attack 10 years from now or not. So that would just be anecdotal belief. There is a little bit of trial evidence. The first study that came out and put some warnings that estrogen might not be the greatest thing for women with coronary disease was a trial called the HERS trial, Heart and Estrogen Replacement Study. They rounded up women who had serious coronary artery disease, and they gave them Prempro on the belief that was the most commonly prescribed hormone back in day, that, “Hey, estrogen is great for the heart. These women will have less heart attacks.” And not only did it not reduce heart attacks, but in the first year, at least in some, there was a little worsening of heart attacks. This is all post hoc analysis of what to make of it. But that was a, “Oh, my God estrogen should have cleared up their heart disease, and it didn’t.”
[00:58:35] But there was a post hoc analysis that showed, and the women with high Lp(a), maybe it did help them. Again, that is just post hoc cherry-picking analysis that might be true, but the only way to prove it to true would be out and go another whole big trial, and women with Lp(a) randomize them to HRT or not and see what happens. And nobody was going to do that trial right there. So, there is that thought there. So, listen, right now, the guidelines would tell you in a woman with heart disease, that is a contraindication for estrogen at any stage. And I think, again, that’s what the guidelines are. That’s what the package insert is. But we all sometimes tiptoe around certain recommendations in certain individuals, and one question would be, if you’re blowing away their ApoB with appropriate therapy, could you be possibly harming them with estrogen or not? I don’t know. You know, you can make the case, look, I normalize all their markers and things are good. So, there’s no good answer for that right now.
[00:59:35] So, in a woman who doesn’t have coronary artery disease, if she had Lp(a), if she didn’t have Lp(a), then I’m making lipid decisions on how high her ApoB or her lipid metrics are. Not that she’s menopausal or not. So, if she had high Lp(a), the guidelines would tell you, “Well, unless ApoB is really normal, that’s a statin person to use there. So, we just don’t have the definitive answers on a lot of these things. And that’s why menopausal women, they’re tricky little creatures, if I could use that word. Listen, I love them. And that was my practice was about. But then I didn’t have the definitive textbook like we had in men that tell you exactly what to do. You were dancing around. And my day talking to you today in 2024, back in the 90s and stuff, we had, like, nothing on women, so we were just assuming they were men and we would treat them accordingly. Not the best way to do things.
Cynthia Thurlow: [01:00:29] No. It’s fascinating to me because I was actually talking to a colleague earlier today and said, “You know, when I finished my nurse practitioner program, around the time of when the WHI came out, I remember women sitting in my office or telling me in the hospital, these are cardiology patients, they were crying because they were taken off of their estrogen. They were progesterone was stopped.” And they said, “My sleep, my energy. I mean, so many factors.” And unfortunately, in many instances, these are women who then had documented coronary artery disease. So for many of them, listening to their stories really left an indelible impression.
Dr. Thomas Dayspring: [01:01:05] Yeah, those were terrible times. And when the study came out and it’s been re-crunched and reanalyzed, even some of the adversity it was seen was you needed a magnifying glass to see the incidence of adversity was there. But yet, once the news people got ahold of this, TV, newspapers, if you’re a woman, you are calling up your doctor, yelling at him, “Why did you ever give me this estrogen?” Like you I said, any number of them. Certainly, the symptomatic ones paid a price by stopping that. And not only the price on how miserably it might be feeling, but who knows? As we’re suggesting now, estrogen in that transition might be good keeping your brain going for your older years and stuff, might be good for the heart if you don’t have existing atherosclerotic disease. So listen, it was a tough time. Look, prior to that estrogen too many doctors, we’re putting it in the drinking water at very high doses for everybody. After that, it disappeared.
[01:02:00] Nowadays, we’re starting to get back into it. But I also know and probably discuss that with you. You know, the gynes are doing it. Gynes are a little shy about heart disease. They’re not as well trained. Internists want nothing to do with HRT, because they don’t want a woman calling up and saying, “Oh, my mom’s spotting or something.” [laughs] That terrifies internists not well schooled in gynecological care. And she’s even nowadays with, I don’t want to get devolved into abortion on this podcast, but spotting in a woman at a certain age is, if you’re in a state where you’re going to go in jail, if you even consider doing something to uterus is tough times.
Cynthia Thurlow: [01:02:38] Yeah, without a doubt. And it’s interesting to me. I’m from a family of very proactive women. And the discussions I have, colleagues and family members that are GYNs, and. And the stories that we go through addressing the needs of all women at different ages and stages, I think that it’s important that we’re having conversations and talking about whether it’s the lack of research or the lack of consistency in terms of how we are addressing women of certain ages.
[01:03:06] If you love this podcast episode, please leave a rating and review, subscribe and tell a friend.



